
(請先閱讀以下研究資料 Please first read the trial information below.)
研究目的
本研究項目旨在探索兩種去氧羥四環素療法於預防男男性接觸者感染細菌性性病的成效。
Aim
This project aims to explore the effectiveness of the two regimens of doxycycline for preventing bacterial STI among MSM.
研究設計
符合資格的參加者將會被隨機分配到兩組:第一組參加者以按需要預防用藥方式(2+1+1)服用去氧羥四環素;而另一組參加者會以事後預防用藥方式(事後24小時內/不遲於72小時)服用去氧羥四環素。
所有參加者需接受合共二十四個月的觀察,期間每三個月自行採集尿液、咽拭子和直腸拭子作性病檢測。血液樣本亦會收集作愛滋病毒及梅毒抗體測試。參加者亦須填答日誌及每月問卷。這是一項開放試驗,參加者將獲悉獲分配的組別。
國際不同研究顯示,相比服用安慰劑,按事後預防用藥方式服用去氧羥四環素能減低感染細菌性的性病的機會。去氧羥四環素在香港是已註冊的藥物,註冊適應症為治療及預防特定細菌感染,而並不包括預防性病感染。因此,服用該藥物作預防性病感染只屬實驗性質。於整個觀察期內,參加者需要填答網上日誌及每月問卷,輸入個人背景資料、可能產生的副作用和性行為及服藥模式。
是次研究的藥物或有一定副作用,包括但不限於頭痛、嘔心、嘔吐、光敏感反應、食慾不振、屙嘔肚痛、紅疹及牙齒變色。參與本項研究沒有可預見的心理,生理,社會,法律,或者經濟危險或得益。假使出現任何相關臨床情況,例如性病感染、嚴重副作用,參加者可以選擇被轉介至醫療服務作醫學跟進。
Study Design
Participants would be randomly assigned into either on-demand pre-exposure prophylaxis (ODPrEP) or post-exposure prophylaxis (PEP) arm.
All participants would be asked to self-collect urine, oropharyngeal and rectal swabs for STI screening every three months during the 24-month observation period. Blood samples would be taken for HIV and syphilis antibody testing. Participants would also be asked to complete a diary and monthly surveys. This is an open-label study.
Multiple clinical trials from different countries have shown that doxycycline taken as PEP could significantly reduce risk of bacterial sexually transmitted infections (STI) compared to placebo. Doxycycline hyclate is a registered pharmaceutical product in Hong Kong. Its indication is for treating and preventing specific bacterial infections, excluding preventing STI. Therefore, taking doxycyline for preventing STI is only experimental. During the follow-up period, an online diary and monthly questionnaires would be administered for recording one’s demographics, followed by the monitoring of reported adverse reactions, and sexual and pill-taking behaviours.
The medicine used in this study may cause side effects, including but not limited to headache, nausea, vomiting, photosensitivity, loss of appetite, diarrhoea, rash, and discolouration of teeth. Participation is entirely voluntary and will not subject the participants to any foreseeable psychological, physiological, social, legal or commercial risks or benefits. If any clinical conditions, e.g. STI, drug reactions, occur requiring medical consultation, referral would be made to specialists service.
本研究中的兩種服藥模式 Two regimens being studied in this trial
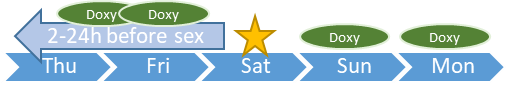
按需要預防用藥 DoxyOnDemand (2+1+1)
性行為前2-24小時服用2粒藥物,在服藥後24和48小時後各服用1粒
若其後性行為持續則每日服藥直至最後一次性行為後的兩日後
Take two doxycycline hyclate 100mg capsules orally 2-24 hours before sex, and one 100mg capsule each 24 and 48 hours after loading dose.
If sexual activities continue, take a daily 100mg capsule until two days after the last sex.
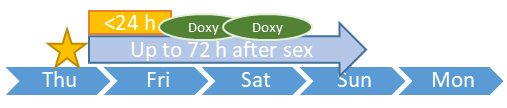
事後預防用藥 DoxyPEP
性行為後的一日內(或不遲於三日)服用2粒藥物
Take two doxycycline hyclate 100mg capsules orally within 24 hours and up to 72 hours after each sex.
時間表
| 月份 | 跟進 |
|---|---|
| 0 | 基線問卷、性病測試*、隨機分配組別、醫學會診及處方藥物 |
| 3 | 性病測試、醫學會診及處方藥物 |
| 6 | 性病測試、醫學會診及處方藥物 |
| 9 | 性病測試、醫學會診及處方藥物 |
| 12 | 性病測試、醫學會診及處方藥物 |
| 15 | 性病測試、醫學會診及處方藥物 |
| 18 | 性病測試、醫學會診及處方藥物 |
| 21 | 性病測試、醫學會診及處方藥物 |
| 24 | 性病測試、醫學會診及處方藥物 |
| Participants are asked to complete an online diary and a monthly questionnaire throughout the study period. | |
| 參加者於研究期間若懷疑感染性病亦可預約到訪進行測試 | |
| *基線檢測性病感染參加者將被邀請完成治療後參與本研究,屆時將由第0月起重新計算 | |
Timetable
| Month | Follow up |
|---|---|
| 0 | Baseline questionnaire, STI tests*, randomisation, medical consultation and prescription |
| 3 | STI tests, medical consultation and prescription |
| 6 | STI tests, medical consultation and prescription |
| 9 | STI tests, medical consultation and prescription |
| 12 | STI tests, medical consultation and prescription |
| 15 | STI tests, medical consultation and prescription |
| 18 | STI tests, medical consultation and prescription |
| 21 | STI tests, medical consultation and prescription |
| 24 | STI tests, medical consultation and prescription |
| Participants are asked to complete an online diary and a monthly questionnaire throughout the study period. | |
| Participants with STI symptoms can make an appointment for STI screening. | |
| *Participants with a baseline STI (CT/NG/Syphilis) positive test result would be invited to join after completing treatment. | |
本研究的性病測試
| 性病測試 | 樣本採集方法 |
|---|---|
| 愛滋病毒抗原及抗體 | 抽血 |
| 梅毒抗體 | 抽血 |
| 淋病 | 尿液、自行採集直腸拭子、自行採集咽拭子 |
| 衣原體 | 尿液、自行採集直腸拭子、自行採集咽拭子 |
| 參加者若確診感染性病可以選擇被轉介至醫療服務作醫學跟進 | |
STI tests in this study
| STI test | Sample collection method |
|---|---|
| HIV antigen and antibody | Venesection |
| Syphilis antibody | Venesection |
| Gonorrhoea | Urine, self-collected rectal swab, self-collected pharyngeal swab |
| Chlamydia | Urine, self-collected rectal swab, self-collected pharyngeal swab |
| Participants diagnosed with an STI would be referred to specialists service. | |
參加資格
參加者須:
- 為十八歲或以上男性、曾與男性肛交
- 經常在港居住
- 參加時沒有感染細菌性性病(若測試顯示感染淋病、衣原體或梅毒,可在治療後再參加;愛滋病感染者及曾感染梅毒而已治癒者皆可參加)
- 能閱讀中或英文及以中或英文溝通
- 具性病感染的風險因素
- 沒有藥物的禁忌症
- 能遵從研究計劃時間表
Eligibility criteria
To join the study, you must:
- be male, aged 18 or above, and have had anal sex with another man; and
- normally reside in Hong Kong; and
- not be infected with bacterial STI at baseline (potential participants tested gonorrhoea/chlamydia/syphilis positive could join the study after completing treatment; people living with HIV and those acquired syphilis before but treated are eligible.); and
- be able to read Chinese and communicate fluently in Chinese/English; and
- be at risk of STI; and
- not have contraindications for the drug; and
- be able to adhere to study protocol.
香港中文大學何善衡傳染病研究中心
S.H. Ho Research Centre for Infectious Diseases, The Chinese University of Hong Kong
電郵地址 Enquiry: hello [at] test4you.online

香港中文大學 – 新界東醫院聯網臨床研究倫理 聯席委員會被授權在有需要時審視個人資料藉以評估研究倫理(參考編號:2023.586)。
如果你有更多關於臨床 研究倫理相關問題,請與該委員會聯繫(電郵 crec [at] cuhk.edu.hk 或 致電 35053935)。
The Joint Chinese University of Hong Kong – New Territories East Cluster (CUHK-NTEC) Clinical Research Ethics Committee is authorized to access the subjects' records related to the study for ethics review purpose (Ref. No.: 2023.586).
For enquiry regarding Clinical Research Ethics, please contact Joint CUHK-NTEC Clinical Research Ethics Committee at crec [at] cuhk.edu.hk and phone at 35053935.