
研究目的
本研究項目旨在探索以訂閱模型提供愛滋病自我檢測套裝以促進男男性接觸者定期進行愛滋病測試。
Aim
The aim of the study is to assess the use of a subscription-based model to distribute HIV self-tests to facilitate routine HIV testing among men who have sex with men.
研究設計
參加者將會被隨機分配到三組:第一組參加者將由第三個月起免費獲得愛滋病自我檢測套裝,第二組參加者將由第六個月起免費獲得愛滋病自我檢測套裝,而第三組參加者將由第九個月起免費獲得愛滋病自我檢測套裝。
參加者在未獲得本研究免費提供的愛滋病自我檢測套裝的時段,會每三個月收到由研究組發出的短訊提醒進行愛滋病測試,參加者在收到信息後的兩星期需填答問卷。
而在獲得本研究免費提供的愛滋病自我檢測套裝的時段內,參加者會每三個月收到由研究組及速遞公司發出的短訊提醒領取愛滋病自我檢測套裝。參加者在收到信息後的兩星期需完成測試、填答問卷並提供測試結果照片作核對。參加者若未能在指定時間內完成自我檢測及問卷,其觀察期將完結。參加者可選擇口腔液或指尖血檢測套裝作愛滋病毒抗體測試。
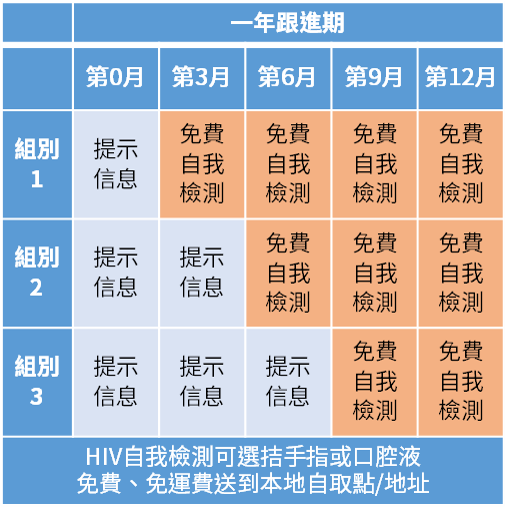
所有參加者需接受合共最多十二個月的觀察,或直至發現感染愛滋病毒,或因上述情況提早完成觀察。參加者完成觀察並填答完成問卷後將會獲贈港幣100元現金券答謝參與。
我們誠意邀請您參加這項研究,參加與否純屬您的個人意願。您可於任何時間退出本項研究。即使您不同意參與,或於其間退出,將不會影響您現時於診所或醫院的診治程序。參加者需提供一個可接收短訊的流動電話號碼作聯絡及獲得取件通知短訊。參加者亦需提供收件地址,或選擇速遞公司提供的自提點地址。參加者提供的流動電話號碼和地址將會轉移至速遞公司作派送愛滋病自我檢測套裝用途。研究過程中,只有參與此項目研究的工作人員才可以閱覽相關記錄;而研究數據將會保留不多於五年。假如您中途退出,有關資料將會被銷毀,不會被記錄和分析。
若檢測結果為陽性,研究團隊會安排抽血作確認測試。假使出現任何相關臨床情況,例如愛滋病毒感染,參加者可以選擇被轉介至公營醫療服務作醫學跟進。參與本研究不涉及任何費用,包括檢測套裝、速遞,以及需要時的抽血確認測試。
Study Design
Participants would be randomly assigned into one of the three arms. Participants in the first, second, and third arm would receive free HIV self-test kits since the third, sixth, and ninth month of the observation period, respectively.
During the period when the participants yet to receive free HIV self-test kits from the Research Team, participants would receive a text reminder from the Team on a trimonthly basis. Participants would be asked to complete an online questionnaire about HIV testing and sexual behavioural profile within two weeks.
During the period when the participants would receive free HIV self-test kits from the Research Team, participants would receive a text message from the Research Team and the courier company reminding to collect the HIV self-test kit. Participants would be asked to complete the HIV self-test within two weeks and the online questionnaire about sexual behavioural profile and HIV testing with a photograph of the self-test result for validation. If the participant did not complete the questionnaire and the self-test, the observation period would finish. Participants could choose from oral fluid or fingerprick self-test kits for HIV antibody screening.
The observation period of all participants would be at most 12 months, unless and until diagnosis of HIV infection, or early completion due to incompletion of the self-test. Upon completion of the observation period and the exit survey, participants will receive HK$100 cash coupon as a token of appreciation.
We sincerely invite you to join the study, the participation of which is however entirely voluntary. You can quit this study whenever you wish. Your access to clinical services would not be affected if you refuse to join the study, or request to withdraw after joining. Participants are required to provide a mobile number which could receive SMS for contacting and receiving the delivery reminder from the courier company. Participants would also be asked to provide an address for collecting the self-test kits. There would be an option to choose one of the pickup points operated by the courier company. The mobile number and address provided would be transferred to the courier company for delivery purpose. All research data would only be accessible to researchers and their staff. Research data would be stored for no more than five years. In the event that you withdraw from the study, study data would be destroyed, and that these would not be recorded or included in the subsequent analyses.
If the screening test result came back positive, the Research Team would invite the participant to perform a confirmation test with venepuncture. If any clinical conditions, e.g. HIV diagnosis, occur requiring medical consultation, referral would be made to specialists in the public service. Participation in this study does not incur cost, including the test kits, delivery service, and the confirmation test when necessary.
參加資格
參加者須:
- 為十八歲或以上的男男性接觸者
- 在港居住
- 能閱讀中文或英文
- 沒有確診愛滋病毒感染
Eligibility criteria
To join the study, you must:
- be a man who have sex with men aged 18 years or above; and
- live in Hong Kong; and
- be able to read Chinese or English; and
- be without known HIV diagnosis
香港中文大學何善衡傳染病研究中心
S.H. Ho Research Centre for Infectious Diseases, The Chinese University of Hong Kong
電郵地址 Enquiry: hello [at] test4you.online

香港中文大學 – 新界東醫院聯網臨床研究倫理 聯席委員會被授權在有需要時審視個人資料藉以評估研究倫理。
如果你有更多關於臨床研究倫理相關問題,請與該委員會聯繫(電郵 crec [at] cuhk.edu.hk)。
The Joint Chinese University of Hong Kong – New Territories East Cluster (CUHK-NTEC) Clinical Research Ethics Committee is authorized to access the subjects' records related to the study for ethics review purpose.
For enquiry regarding clinical research ethics, please contact the committee at crec [at] cuhk.edu.hk.